Cyclic Voltammetry
Cyclic voltammetry (CV) is a method of measuring electrochemical processes. It involves a counter electrode and a reference electrode set up at a specific potential, or potential range. All electrochemical processes take place with reference to this reference potential, so the reference electrode is a crucial part of the setup. The working electrode changes from a solid state to a solution and back again, and all of these processes take place relative to the reference electrode’s potential.
Spectroscopy
Spectroscopy of cyclic-voltammetry is a versatile technique used for examining electroactive materials. It provides information about redox properties, doping mechanisms, stability, and energy storage. It also allows for the estimation of electron affinity and ionization energy. Also it is more affordable than many other high-vacuum techniques. It measures changes in electron mobility through correlation with energy levels of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO).
The EPR spectroscopy of manganese(II) enables detection of materials with unpaired electrons. The spectra can also help in the localization of radicals. Because electrons have a spin, their spins can either be parallel to or antiparallel to a magnetic field. This is known as the Zeeman effect, and it is a useful method for determining the localization of radicals.
The CV scan should be performed using an initial potential of -0.5 to 1 V. Then, a series of scans at 25 mV/s should be performed. The scan rate can be varied depending on the slope of the excitation signal. If the results are negative, the contamination may be caused by compounds with low oxidation potential, such as glass particles.
The CV spectroscopy technique can be used to study the chemical reactions in a biological system. It enables scientists to analyze the speed of electron transfer through the onset of the reactions. It can also be used to analyze the electrochemical reversibility of reactions. The results can help establish whether a reaction is irreversible or reversible.
Molecular spectroscopy
Molecular spectroscopy using cyclical voltammetry is a versatile technique that is useful for a variety of applications. This method is particularly useful for studies involving the transfer of electrons, which are essential for most processes. It can also be used to study the mechanisms and characterization of substances. Its versatility has made it an essential part of the grand project of chemistry.
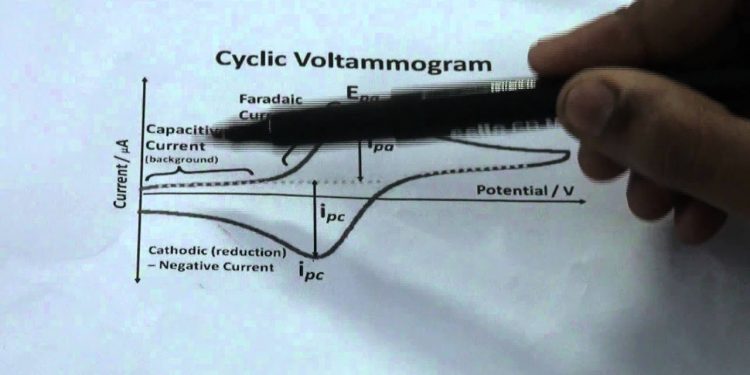
The basic principle of cyclic voltammetry is that the peak current of a substance depends on the scan rate. The relationship between peak current and scan rate is defined by the Randles-Sevcik equation. In addition, cyclic voltammetry samples a small amount of a sample solution. The sample is obtained in the diffusion layer of an electrode. Moreover, the analyte must be redox active.
The redox potential is increased by increasing the scan rate. In ideal conditions, the peak width is 90mV. As the scan rate increases, the peak current is proportional to the oxidant concentration. Increasing the scan rate can measure the rate of interfacial electron transfer and coupling reactions. In addition to measuring the concentration of analyte, cyclic voltammetry can also be used to measure the antioxidant capacity of a sample.
Cyclic voltammetry is a versatile technique for analysing organic molecules. In particular, it has been used for glucose detection and the detection of other organic compounds. This technique is also useful for studying electroactive species, explosives, and nitro compounds. Modern data analysis methods enable the identification of pesticides and trace amounts of metabolites.
Electrochemical spectroscopy
Cyclic voltammetry and electrochemistry are a pair of chemical analysis methods. In both methods, the analyte is a solution and an electrode is placed in it. The voltammogram produced by these methods is proportional to the square root of the scan rate, as explained by the Randles-Sevcik equation. The cyclic voltammetry technique is particularly useful when the analyte is redox active.
Electrochemical spectroscopy and cyclic voltammetry are important tools for studying the reactivity of inorganic compounds. They help understand how electron transfer processes take place. In addition, voltammograms can be used to estimate intramolecular decomposition rates.
Cyclic voltammetry is a powerful tool for analyzing complex samples. Its ability to measure cross-selectivity and high sensitivity enable scientists to measure the presence of biomolecules in a wide variety of biological systems. Electrochemical spectroscopy and cyclic voltammetry are complementary techniques that can be combined to make better and more sensitive measurements.
Cyclic voltammetry works by measuring the voltage change at an electrode. It uses a standard called ferrocene (Fc) as an internal standard. When the electrode potential increases, ferrocene (Fc) loses an electron. This movement of electrons produces an electrical current that can be measured with the help of a potentiostat.
Cyclic voltammetry is one of the most common electroanalytical chemistry techniques. It is inexpensive, easy to use, and provides accurate information about redox reactions and the kinetics of heterogeneous electron-transfer reactions.