G0 to G1
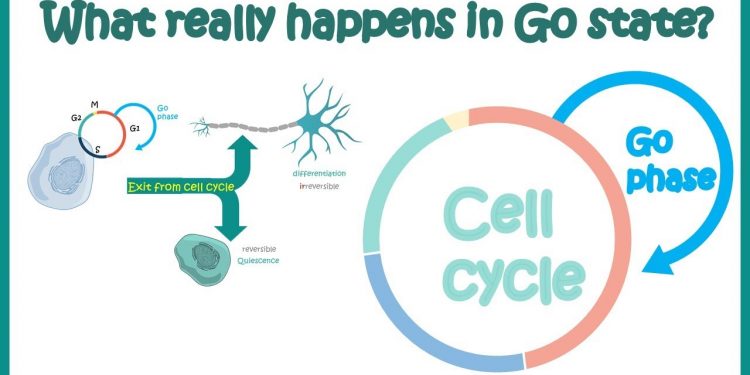
The G0 phase is the cellular state outside of the replicative cell cycle. Environmental factors, such as deprivation of nutrients and resources for proliferation, are believed to cause a cell to enter G0. The G0 phase is therefore a state of rest. This article will explain the process that occurs when a cell transitions from G0 to G1.
Cell cycle
The G0 phase of the cell cycle refers to a period during which a cell is in a quiescent state and not dividing. Many scientists consider this phase to be an extension of the G1 phase, during which cells prepare to divide. However, some cells enter the G0 phase permanently, for instance parenchymal cells. These cells are only able to enter the G0 phase when certain conditions are met.
To determine whether a cell is in the G0-phase, it was found that cells had lower RNA content than cells in the S or G2/M phases. Researchers have used this information to distinguish between the two phases of the cell cycle. During the first phase, cells synthesize RNA and proteins. In the second phase, cells produce more DNA. The difference between the two phases is the time taken for a cell to reach that phase.
The cloning process reduces the number of immortal stem cells and mutagenic risk. Moreover, errors occur mostly in the cells that will die within four months. The replacement kinetics of each renewable tissue play a crucial role in the fraction of stem cells that are in G0. The replacement kinetics of cells in G0 have been studied extensively in the Radiobiological literature. In particular, it is important to know how long a stem cell can remain in G0 in order to ensure that they do not die.
While a cell may arrest before it enters the S phase, a transition is required in order to complete the G1/S phase. This transition is known as the Start checkpoint in budding yeast. If a cell cannot undergo coherent gene expression, it may prolong G1. Alternatively, the cell can progress through the rest of the cell cycle. The G1/S transition regulates the activity of several proteins that regulate the cell cycle, including the Cyclin A/Cdk1, the transcription factor Cdc28, and the transcription inhibitor Whi5.
Expression of mVenus-p27K-
One of the main functions of mVenus-p27k is to detect quiescent cells in skeletal muscle. These cells are composed of a large population of myogenin-positive cells, which express markers of muscle stem cells. Interestingly, mVenus-p27k is not expressed in most neural cells, which are thought to be a poor source for stem cells.
The reason for the limited proliferation of these cells could be due to quiescence. The corresponding G0 phase is the last stage of the cell cycle, and quiescent cells are thought to exit the cell cycle at this point. To test this, HEY1 cells transduced with mVenus-p27K and mCherry-CDT1 vectors were used. These two markers are useful in defining the G0/G1 transition. They also help to define the G1/S phase. Thus, cells with low expression of mVenus-p27K-expression are likely to be in the S/G2/M phase.
Cells expressing mVenus-p27K were enriched by contact inhibition and serum starvation. In addition, these cells displayed typical G0 features, including low Ki67 expression and reduced phosphorylated retinoblastoprotein (pRb).
These data suggest that mVenus-p27K’s can identify quiescent cells in the S/G0/G1 cell cycle. However, further studies are required to determine whether this fusion protein can distinguish between quiescent and proliferating cells. However, these results suggest that quiescent cells are more easily distinguished in the G0/G1 phase than their counterparts.
The retinoblastoma protein physically interacts with the anaphase-promoting complex, preventing neuronal proliferation. This inhibits cyclin-dependent kinase (Cdk) activity. This protein can also inhibit tumour growth by suppressing the G1/S inhibition of the cell cycle. In addition, mVenus-p27K inhibits Rb-E2F switch, preventing cell death.
Replication stress
The replication stress response is a cellular mechanism that helps DNA replication machinery overcome DNA lesions and intrinsic replication fork obstacles, and ensure faithful transmission of genetic information to daughter cells. The recent identification of multiple pathways involved in replication stress has raised questions about their distinct functions and whether they may be redundant. Here, we review the mechanisms involved in replication stress in mammalian cells and how they may impact the dynamics of the core DNA replication complex.
The Replication-Stress Response is induced when the replisome fails to finish the replication process. This leaves a gap in the ssDNA, which is normally filled by an error-free HDR process or by specialized TLS polymerases. This unrepaired ssDNA gap may also convert into a DSB. Overtime, excess ssDNA gaps could overwhelm repair mechanisms in G2, resulting in chromosomal aberrations.
The activation of proinflammatory pathways in CD4+ T cells in older adults is due to replication stress. The naive CD4+ T cells from old adults possessed enriched gene sets associated with cellular senescence. Moreover, these CD4+ T cells lacked the senescence-associated secretory phenotype when resting, indicating that this response is triggered by a proliferative response.
Cell cycle transition from G0 to G1
The cell cycle transition from G0 to the G1 phase is an important step in the life cycle. The G0-G1 transition occurs just prior to the S-phase progression in the PT and DT. During this transition, many changes occur in gene expression. Several factors can influence this transition. In this article, we will examine several of these factors. This will help you understand how the transition from G0 to G1 affects your cell’s life cycle.
The G1 phase occurs when the cell reaches the growth phase. This phase occurs when the cell is biochemically active, accumulating the building blocks of chromosomal DNA and the associated proteins. During this phase, cells also decide whether to divide into two daughter cells. The M phase is also a crucial point in the cell cycle, because this is where chromosomes replicate and organelles are produced.
The PT phase cells accumulate rapidly before the S phase progresses. This rapid accumulation of G1 cells may be triggered by an injurious or proliferative stimulus. The PT cells’ G0/G1 ratio was higher than in DT cells. The UA-treated rats did not undergo a prompt G1-S transition after a proliferative stimulus. Moreover, the G0-G1 transition occurs before the S-phase progression. In PT cells, the G0-G1 transition occurs before the S-phase progression. The PT cells accumulate more G1 phase cells than DT cells, and they undergo repair of DNA damage before the S-phase.
The cell cycle is essential for tissue growth. It is organized into four distinct phases, which are governed by checkpoints. Loss of cell cycle control is one of the factors associated with cancer. A loss of these checkpoints can lead to a cell’s failure to repair its DNA. This is what causes cancer. So, what are some of the changes in cell cycle regulation? And how do they affect cancer?
Cell cycle transition from G0 to S phase
The transition from G0 to S phase occurs from the checkpoint during G1 phase. This occurs when cells have exhausted the nutrients and growth factors required for cell division. The cyclin-CDK complex deactivates Cdh1 and activates the expression of components of S-phase cyclin-CDK. The S-phase cyclin-CDK complex initiates the cell cycle and moves it to the next phase. This process is governed by a signaling cascade. Cyclins are conserved in each group; however, the specific function of each CDK may differ.
The duration of G0 is eight hours in cells treated with siRNA. The ratio of DNA to RNA in stained cells remains low during this period, indicating that the G0-G1 transition is correctly identified. The p27 protein, a transcription factor in the cell cycle, is expressed by a subset of cells during the G0-G1 phase. This may facilitate a faster cell cycle and lead to apoptotic cells.
A checkpoint called G1/S determines whether a cell is ready to enter S phase. This checkpoint controls the cell’s nutritional status and DNA integrity. This checkpoint is particularly important in cells that are about to enter S phase. The size of the cell can be used as an indication of readiness to enter the S phase. In yeast cells, for example, G1 checkpoints signal when a cell is ready to enter S phase.
The G1/S transition is regulated by cyclins. Cyclin A, Cyclin D, Cyclin E, and Cyclin D are active in the S phase, while Cyclin E regulates the transition from G1 to S. There are also G2/M cyclins required for the transition from G1 to S phase. These proteins are synthesized during G1 and degraded at the end of the M-phase.